Investigating targeted delivery of pro-repair factors to the lungs
About the Project
3-year NHLI-funded PhD post-starting Spring 2023
Summary of Research
Applications are invited from candidates with a Masters degree and undergraduate training in a biological science or bioengineering discipline for a PhD to investigate targeted delivery of pro-repair factors to the lungs.
The studentship will be funded for three years with a tax-free bursary of £19,668 per annum plus tuition fees. This studentship is based in the National Heart and Lung Institute at Imperial College London and will be supervised by Dr Charlotte Dean and Dr Matthew Hind.
The lungs are capable of intrinsic repair however, in some people, disruption of these repair processes leads to disease caused either by lack of repair, or an overactive repair response. There are no curative treatments available for many prominent lung diseases including Chronic Obstructive Pulmonary Disease, Adult Respiratory Distress Syndrome and Bronchopulmonary Dysplasia.
Regenerative biology now offers real therapeutic potential to repair or regrow damaged lungs. The aim of the lung development and repair group is to identify and develop regenerative medicine treatments for the lungs that can be used to repair damaged lung tissue and ameliorate diseases.
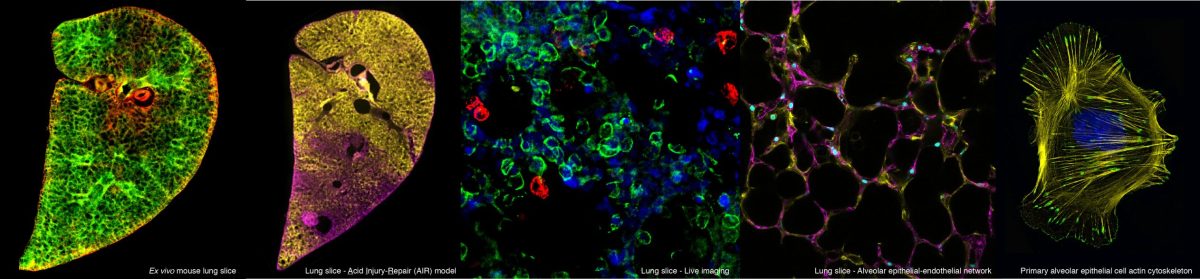
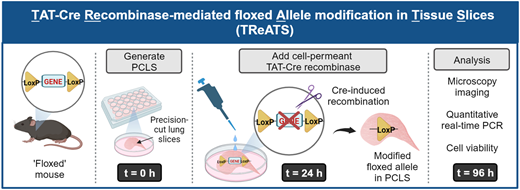
This PhD project will investigate strategies to target delivery of pro-repair treatments to the lungs. The student will investigate methods to encapsulate pro-repair factors in nanoparticles or hydrogels to extend the efficacy of the pro-repair signals and allow their precise targeting to the lung alveoli. The project will utilise a variety of cutting-edge models, including 3D lung slices to investigate ways to combine potential repair treatments with bioengineering approaches to stimulate optimal lung repair.
The student will be based in the Cardio-Respiratory Section within the National Heart and Lung Institute, which provides an exciting environment, with state of the art facilities and excellent opportunities for PhD student training including research seminars. All students will belong to Imperial’s award-winning Graduate School which provides a comprehensive Professional Skills Programme.
How to Apply
Applicants must hold, or expect to obtain, a first or upper second-class undergraduate degree or UK equivalent, along with a Masters, both in an appropriate subject from a recognised academic institution.
To apply please email the following information to c.dean@imperial.ac.uk with:
- Curriculum Vitae (max 2 pages)
- Personal statement (1 page)
- Name, address, telephone number or email of two referees. At least one of which must be academic.
Applicants unable to attend interview in person will undergo an online interview and be invited for a second face-to-face meeting before confirmation of offer.
Eligibility and funding notes
This studentship is open to home and international students
The successful candidate will receive a bursary of £19,668 per annum plus tuition fees for home students. Successful non-UK students will be offered a bursary with a contribution of £20,000 p.a. towards international tuition fees and will be expected to cover the remaining fees themselves.
Please note that candidates must fulfil College admissions criteria.
Application deadline: 30th November 2022

![]() Additionally, our contribution has also planted a tree in The Forest of Biologists.
Additionally, our contribution has also planted a tree in The Forest of Biologists.